What is Rivfloza®
(nedosiran) injection?
A once-monthly treatment that lowers and keeps urinary oxalate levels under control in people with PH1.
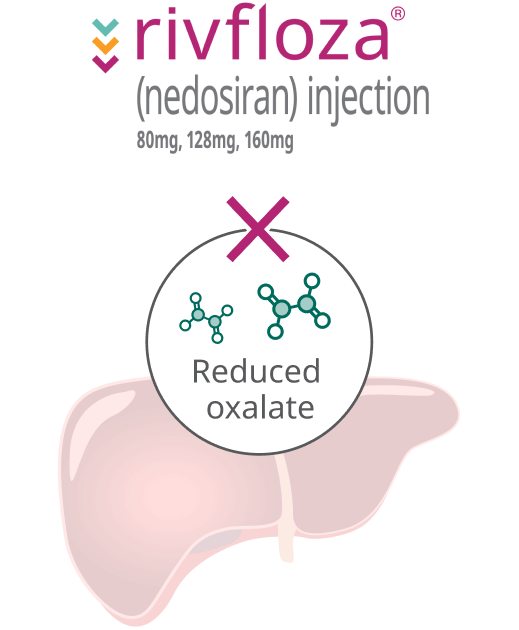
Rivfloza® helps prevent overproduction of oxalate in the liver
How Rivfloza® works
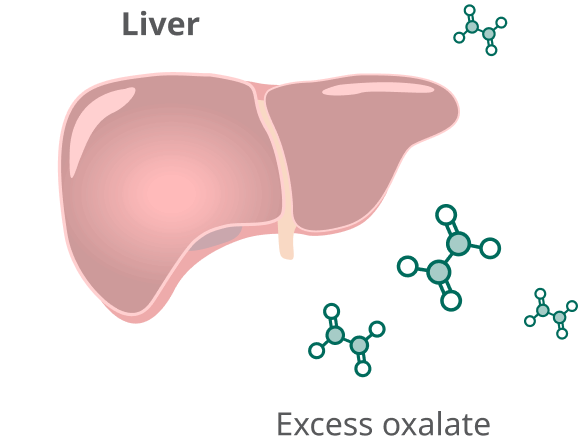
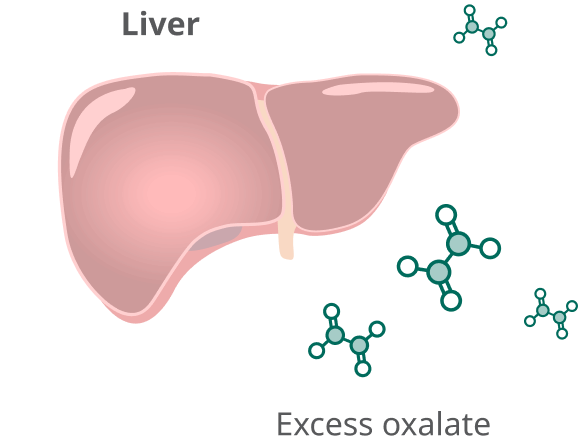
In people with PH1, the liver produces too much oxalate.
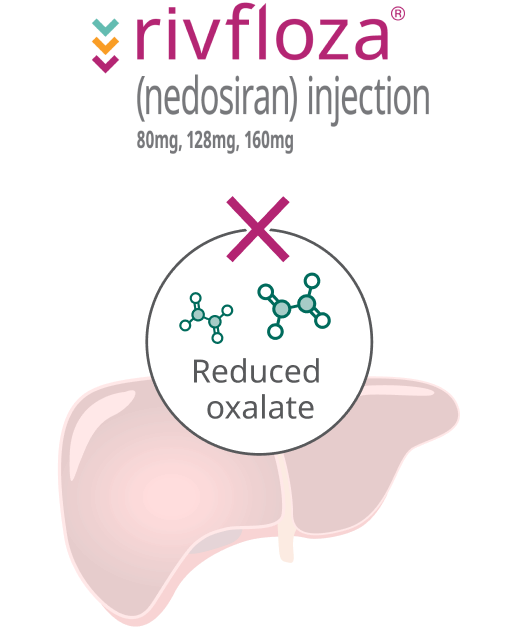
Rivfloza® targets the liver to reduce the overproduction of oxalate.
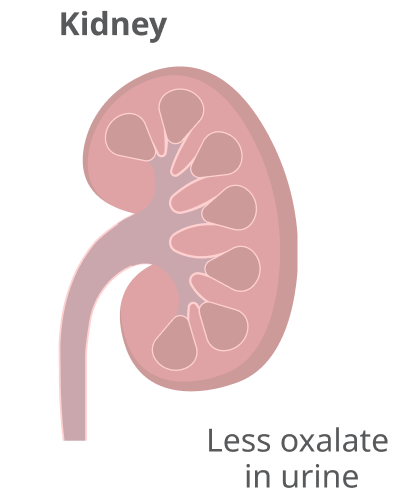
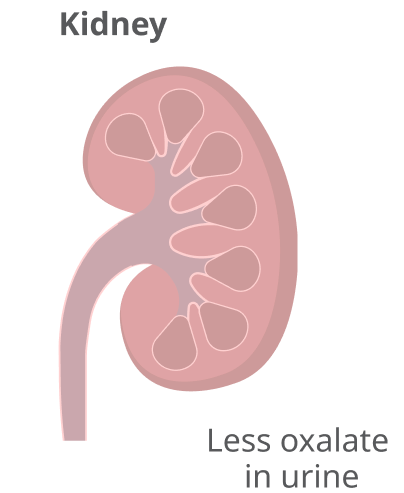
The result is lower levels of oxalate in urine, which is eliminated by the kidneys.
Rivfloza®’s effectiveness and safety are based on 2 different trials of PH1 patients with relatively preserved kidney function
How Rivfloza® was studied
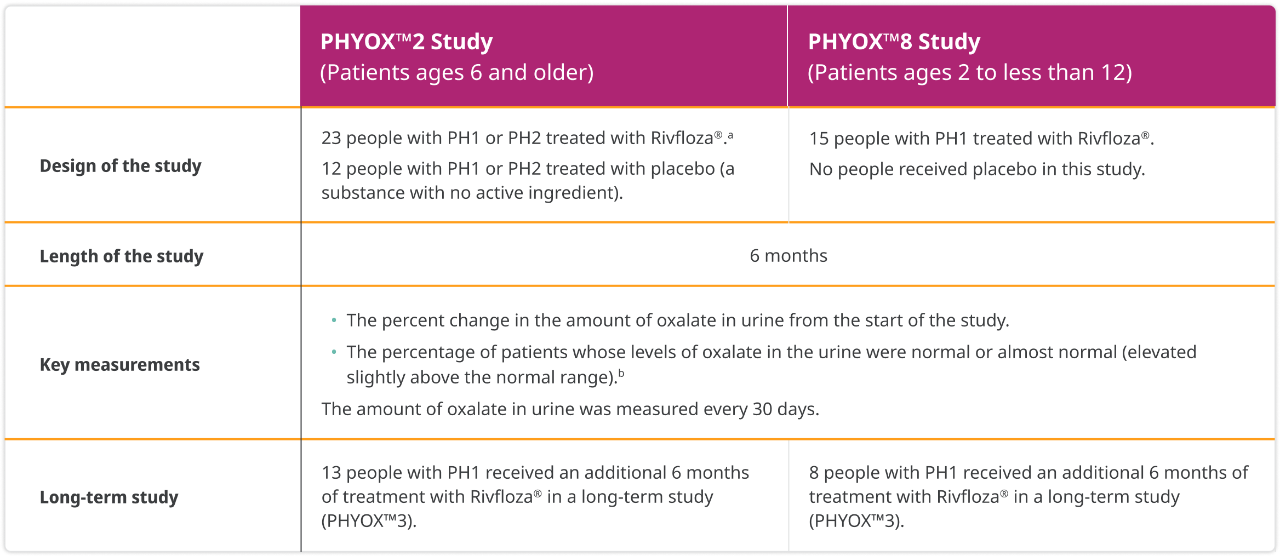
aToo few people with PH2 were enrolled in the study to evaluate the effectiveness of Rivfloza® in PH2. Therefore, Rivfloza® is only approved for use in people with PH1.
bIn PHYOX™2, people who received placebo in the study had higher initial urinary oxalate levels.
Rivfloza® reduces oxalate levels in the urine and keeps them under control
In PHYOX™2:
People with PH1 treated with Rivfloza® achieved an average difference of 56% in 24-hour urinary oxalate compared to placebo, measured monthly from 3 months to 6 months.
3 out of 4 people with PH1 treated with Rivfloza® had reduced levels of oxalate in their urine, down to normal or almost normal levels after 6 months of study.
In PHYOX™8:
Children with PH1 treated with Rivfloza® achieved a 64% average reduction in spot urinary oxalate:creatinine ratio, measured at 6 months.
93% of people with PH1 treated with Rivfloza® had an almost-normal urinary oxalate:creatinine ratio on at least one visit over 6 months.
Across both PHYOX™2 and PHYOX™8 studies: All 21 people who received Rivfloza® for an additional 6 months maintained the reduction in urinary oxalate in the long-term study.
Talk to your health care professional about what to expect from treatment.

Rivfloza® is safe and well-tolerated
There were no serious treatment-related side effects in people with PH1 who received Rivfloza® in the clinical studies.
Most common side effects (occurring in ≥20% of patients treated with Rivfloza®) were injection site reactions (ISRs).
- In PHYOX™2 (patients ages 9-46), 39% of patients experienced ISRs.
- In PHYOX™8 (patients ages 2-11), 13% of patients experienced ISRs.
- These ISRs included reddening, pain, bruising, rash, or dimple at site of injection.
- They were generally mild and did not lead to stopping treatment.